This trial is now closed
Welcome to the patients' area for the TACTIC-E trial. This trial is recruiting people who have been hospitalised with confirmed COVID-19 disease. The purpose of this trial is to prevent organ damage and reduce the need to transfer patients to ICU and ventilation.
The following information will provide answers to questions you might have about this trial.
- What is the purpose of the trial?
-
COVID-19 was declared a pandemic on 11th March 2020 by the World Health Organisation (WHO). It is a disease affecting the lungs and is caused by a new coronavirus known as SARS-CoV2. Most people with the virus have mild symptoms. However, some people, for example, older adults and those with underlying health conditions including heart disease and diabetes, may develop more severe symptoms, leading to hospitalisation, increased support for breathing (e.g. use of a ventilator in an intensive care unit) or even death. There are currently no vaccines against the virus, or proven treatments to treat it.
Some of the severe symptoms of the virus are thought to be a result of an overactive immune response, leading to organ damage in the body. The purpose of this trial is to determine the best way to treat patients with COVID-19 infection by comparing different treatments which act on the immune system, with the aim of reducing severe symptoms and therefore the number of Intensive Care Unit (ICU) admissions in hospital.
- What are the drugs being tested?
-
The treatments are:
- EDP 1815 is a microbe found in human intestines which is thought to have a calming effect on an overactive immune system
- Ambrisentan and Dapagliflozin are two different drugs, each taken as a single capsule once daily. Ambrisentan is a licensed drug which targets the walls of blood vessels in the lungs and blunts inflammatory activity in the lungs. It is commonly used to treat a condition known as pulmonary arterial hypertension. Dapagliflozin is a licensed drug which helps the kidneys excrete glucose and it is commonly used in type 2 diabetes mellitus.
All trial participants will receive standard of care. Both types of trial treatments are being compared to being on standard care only. Some patients will be randomised to standard care arm only (which may include standard antivirals), that is without any new drug.
TACTIC-E will use a platform design. A "platform trial" is a clinical trial with a master protocol, which allows for multiple treatments to enter or exit the trial over the course of the study. This means that the trial team can, with interim analyses, stop recruiting to treatment groups (arms) early where a clear early decision can be made. It also allows for the addition of further arms and treatments. If the treatment you have been assigned to is stopped, you will immediately stop treatment.
- Who is being invited to take part?
-
Patients may be invited to participate in this trial if they are or are suspected to be COVID-19 positive, are considered to be at higher risk of developing serious symptoms, and where it is believed Ambrisentan and Dapagliflozin or EDP1815 may be suitable treatments.
We plan to include up to 469 patients (in each arm) with COVID-19 disease from a number of hospitals across the UK.
- Do I have to take part?
-
No, participating in this trial is completely voluntary. If you do decide to participate you will be asked to sign an Informed Consent Form, however, you are still free to change your mind and leave the trial at any time without giving a reason. If you choose not to participate or to leave the trial, your future medical treatment will not be affected in any way.
- What will happen to me if I take part?
-
The following flowchart summarises the trial:
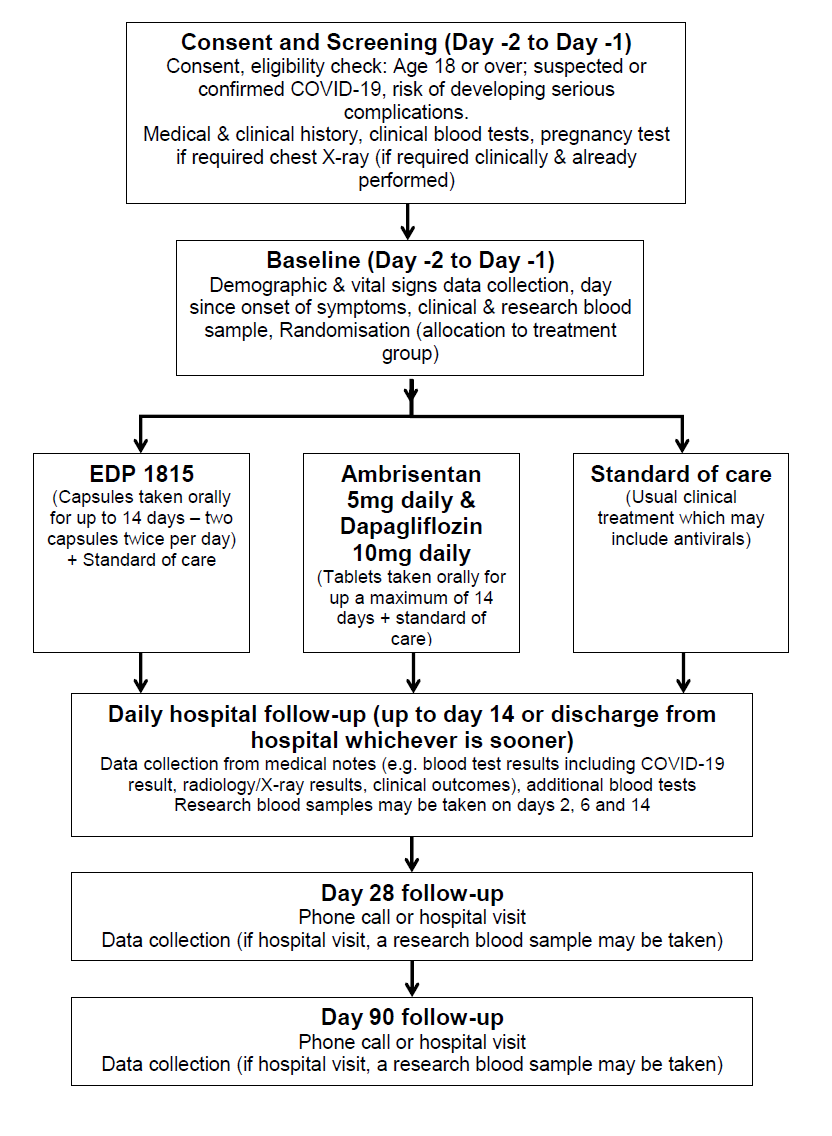
There are 3 groups in this study; one group gets the normal treatment (standard of care) and two groups which will each receive a different treatment. As we don’t know whether EDP 1815 or a combination of Ambrisentan and Dapagliflozin on top of standard of care can be used to treat COVID-19 related disease, we need to compare the treatments separately to the regular standard of care. The trial treatments mentioned above will be in addition to your usual clinical care. Please note, all assessments up to day 14 are conducted at the hospital either up to day 14 or up until discharge from hospital whichever is sooner.
Consent and screening (approximately 45 minutes): If you agree to participate in the trial, you will sign the Informed Consent Form at the end of this document and be given a copy of this to take away and refer to later. The screening assessments include checking your clinical and medication history, checking that you are suitable for the trial, and reviewing blood tests you have had since admission to hospital. If the blood tests have not yet been done, these will be taken and checked. The screening process may happen up to 48 hours before trial treatment begins. However, since COVID-19 requires urgent treatment, where urgency is needed, screening and baseline procedures can happen on the same day. If you are able to understand but unable to physically sign the consent form, either a family member or independent doctor can act as a witness and sign the consent form to attest that the information was accurately explained to, and apparently understood by you.
Baseline (duration, approximately 45 minutes): Further routine clinical information from your medical notes will be collected. You will also be invited to give a blood sample (blood group, serum and DNA/genetics biomarkers, sample for future analysis; the maximum amount of blood taken will be approximately 30ml [2 tablespoons]). If you are suitable for this trial, you will be randomised to a treatment group.
Randomisation: Brief details identifying you and the answers to a few questions about your health and medical conditions will be entered into a computer. The computer will then allocate you at random (like rolling dice) to one of the possible treatment options. In all cases, the treatment groups will include the usual standard of care for your hospital. This trial will randomise patients in a 1:1:1 format. This means that for everyone patient randomised into the standard of care arm, one patient will be randomised into each of the other treatment groups.
Treatments and In-hospital follow-ups (days 1-14, or up to discharge from hospital, whichever is sooner): Following random allocation to a treatment group, treatment will commence. The options are:
EDP 1815 (an unlicensed product (human-derived bacterium) which is being developed for the treatment of inflammatory diseases; it acts by modulating the immune system) Ambrisentan and Dapagliflozin (Ambrisentan is a licensed drug which targets the walls of blood vessels in the lungs and blunts inflammatory activity in the lungs. It is commonly used to treat a condition known as pulmonary arterial hypertension. Dapagliflozin is a licensed drug which helps the kidneys excrete glucose and it is commonly used in type 2 diabetes mellitus) Standard of care (usual clinical care for COVID-19 infection which may include antivirals)
We will collect information from your medical notes about how you are doing, your vital signs, what blood tests and x-rays/scans you have had during your hospital stay, and the results of these. Depending on the group you are in we may run additional tests to monitor the acid levels in your blood. On days 2, 6 and 14, research blood samples may also be taken, to look at immune cells and markers in the blood. We will take a maximum of 30ml of blood (2 tablespoons).
Day 28 and day 90 Follow-up (Telephone call or hospital visit): We will be asking a few questions about your health and collecting data from your medical notes. If you visit the hospital we may also take a further research blood sample and collect data from other clinical tests such as chest X-rays or lung function tests if they are performed as part of your standard of care.
- What will I have to do?
-
This trial will run during your stay in hospital. Depending on the treatment group you are allocated to, the drugs have different dosing regimens. These are described briefly in the trial flowchart, and in more detail below:
EDP 1815
This drug is taken as capsules orally for 7 days; the trial doctor may extend your treatment up to 14 days – if it is felt that you are responding to treatment. You will be taking two capsules twice a day. Each dose should be on empty stomach e.g one hour before or two hours after food. There should be at least two hours between your twice-daily doses. If you improve and are discharged before this time, the treatment will be stopped.
Ambrisentan
This drug is taken as a tablet once a day for up to 7 days; the trial doctor may extend your treatment up to 14 days if you remain as an inpatient if it is felt that you are responding to treatment. The tablet may be taken with or without food and is to be swallowed whole. If you improve and are discharged before this time, the treatment will be stopped.
Dapagliflozin
This drug is taken as a tablet once a day for up to 7 days whilst you are an inpatient; the trial doctor may extend your treatment up to 14 days if you remain as an inpatient – if it is felt that you are responding to treatment. The tablet may be taken with or without food and are to be swallowed whole. If you happen to be randomised to this arm, the acid levels in your blood will be monitored. If you improve and are discharged before this time, the treatment will be stopped.
You should tell the trial team if you feel unwell or different in any way. If you have any major concerns or are feeling very unwell please contact your trial doctor immediately. If you have been discharged from hospital please contact the trial doctor using the contact numbers at the end of this information sheet.
Following discharge from the hospital, we will ask you to attend or be available to talk on the phone for your day 28 and day 90 follow-up visits. If these visits happen at the hospital we may ask for further clinical and research blood samples.
You should discuss your participation in this trial with any insurance provider you have (e.g. protection insurance, life insurance, income protection, critical illness cover and private medical insurance) and seek advice if necessary, as failure to notify them may affect or invalidate your cover.
- What are the side effects of the drugs?
-
EDP 1815
No specific side effects have been described with EDP 1815.
Ambrisentan
Very common (more than 10 in 100 of patients): headache, peripheral oedema, fluid retention
Common (less than 10 in 100 of patients): anaemia, dizziness, cardiac failure, palpitations, low blood pressure, flushing, nosebleeds, difficulty breathing, upper respiratory congestion, nausea, vomiting, diarrhoea, abdominal pain, constipation, increased liver enzymes (transaminases), chest discomfort or pain, lack of energy, fatigue.
Uncommon (less than 1 in 100 of patients): hypersensitivity reactions, e.g. angioedema (swelling under the skin) rash, itchiness, fainting, autoimmune hepatitis, liver injury
Dapagliflozin
Very common (more than 10 in 100 of patients): hypoglycaemia (low blood sugar when used with insulin or sulphonylurea drugs).
Common (less than 10 in 100 of patients): genital infections, urinary tract infections, dizziness, rash, back pain, painful or difficult urination, increased urine output, blood test results which show an increase in haematocrit (increase in the volume of red blood cells in your whole blood), decrease in creatinine renal clearance, which indicates kidney function, or dyslipidaemia (changes in the fat concentrations).
Uncommon (less than 1 in 100 of patients): dehydration, hypotension and thirst, dry mouth and constipation, nocturia, genital pruritus, and blood test results which show an increase in blood creatinine and urea levels, which indicate kidney function and decreased weight.
Rare (less than 0.1% of patients): diabetic ketoacidosis
Very rare (less than 0.01% of patients): Fournier's gangrene, angioedema (swelling under the skin)
- What are the possible disadvantages and risks of taking part?
-
Blood tests: Occasionally, some bruising or inflammation at the needle site may occur and very rarely infection at the puncture site.
Drug treatment: Both Ambrisentan and Dapagliflozin are given to patients in the UK and are well tolerated. However, individual patients can react differently to a particular drug which means that there is a possibility of experiencing side effects. The trial doctor will monitor any side effects regularly and take appropriate actions where necessary. In this trial, ambrisentan is administered in conjunction with dapagliflozin (two different types of the drug) both to offset any negative effects of fluid re-distribution in those with COVID-19 and because patients may derive further benefit from dapagliflozin therapy.
There are no clinical data available on the effects on pregnancy for either Ambrisentan or Dapagliflozin. However, animal studies of Ambrisentan have shown toxicity to the foetus and animal studies of Dapagliflozin suggest toxicity to the foetus. Ambrisentan is contraindicated in pregnancy and Dapagliflozin is not recommended to be taken by pregnant women.
EDP 1815 is a single strain of a bacteria which is often found in your gut. EDP 1815 does not cause infections, is not genetically modified, does not persist in the gut, and does not alter the normal bacteria in your gut. EDP 1815 acts locally in the gut without being actually absorbed but has effects throughout the inside of the body modulating your immune system without getting into the blood. These medicines are therefore very well tolerated. One of the key reasons for selecting EDP 1815 as a treatment arm in this study is due to its safety profile, which would be particularly beneficial in the COVID-19 population as there is no evidence that it increases the risk of infection due to its novel and unique mechanism of action. EDP 1815 is currently in phase 2 clinical development and has European and US approval to initiate a multinational psoriasis study, scheduled for the second half of 2020. There are no clinical data available of the effects in pregnancy for EDP 1815.
You should not participate in this trial if you are planning to become pregnant or father a child during the trial. Women who are able to have a baby must use one of the following reliable forms of contraception for the entire duration of the trial (90 days) after upon completion of the last treatment. This includes:
Intrauterine Device (IUD) Hormonal based contraception (pill, contraceptive injection or implant etc.) Barrier contraception (condom and occlusive cap e.g. diaphragm or cervical cap with spermicide) True abstinence (where this is in accordance with the participants’ preferred and usual lifestyle)
If you or your partner becomes pregnant during the trial (within 90 days) after completion of the last treatment, you should inform your trial doctor immediately.
You should discuss your participation in this trial with any insurance provider you have (e.g. protection insurance, life insurance, income protection, critical illness cover and private medical insurance) and seek advice if necessary, as failure to notify them may affect or invalidate your cover.
Men are required to use adequate contraception for the entire duration of the trial (90 days) upon completion of the last treatment. This includes:
Barrier contraception (condom and spermicide) even if the female partner(s) are using another method of contraception or are already pregnant (also to protect male partners from exposure to the trial IMPs etc.) True abstinence (where this is in accordance with the participants’ preferred and usual lifestyle)
- What are the possible benefits of taking part?
-
There is no guarantee that you will benefit from taking part in this trial. However, information collected as part of your participation in this trial may benefit patients with COVID-19 in the future.
- What are the alternatives for treatment?
-
There are no known preventative drugs for COVID-19 currently.
- What will happen if become very ill and I can longer take the tablets?
-
If you become very unwell or confused during the trial but can continue to swallow the treatment tablets, we will continue with the treatment and blood tests. When you are better we will check that you did want to and wish to stay in the trial.
If you become so unwell that you cannot swallow the treatment tablets your participation in the trial will stop. We will keep the information that we have collected about you up until this point, but not after this point
- What happens when the trial stops?
-
Once the trial has ended you will be referred back to regular treatments. Pending the results of the trial, treatment guidelines may change.
- Will I receive any expenses or payment?
-
You will be reimbursed travel expenses for any research visits which require you to attend a hospital visit after you have been discharged.
- Optional endothelial cell collection
-
In some centres, we are also conducting a smaller study for patients participating in the main study. We will provide you with an additional information sheet and consent form describing an optional procedure called Endothelial Cell Collection. You can still take part in the TACTIC-E trial if you choose not to take part in the endothelial cell collection.
- What if new information becomes available?
-
Sometimes during the course of a trial, new information becomes available which might affect your decision to continue participating in this trial. Your trial doctor will contact you to discuss the new information and whether you wish to continue participating in the trial. If you still wish to continue on the trail, you will be asked to sign a new Informed Consent Form. The trial sponsor, the regulatory authority or the trial doctor may decide to stop the trial at any time. If that happens we will tell you why the trial has been stopped and arrange for appropriate care for you.
- What if I decide I no longer wish to participate in the trial?
-
You are free to come off this trial at any time without giving a reason and without affecting your future care. If you decide not to participate any further, you will no longer receive the trial treatment. No further tests will be performed on you and no further research samples will be collected. Any data already collected or results from tests already performed on you or your samples will continue to be used in the trial analysis.
The trial doctor may also choose to withdraw you from the trial if they feel it is in your best interests or if you have been unable to comply with the requirements of the trial. Reasons for trial withdrawal could include:
- You have experienced a serious side effect
- You are unable to complete the visits, medication or trial documentation as required
- The trial doctor feels you no longer appear to benefit from the treatment
If you have experienced any serious side effects during the course of the trial which require you to withdraw from the trial, your trial doctor will follow-up with you regarding your progress until the side effect has stabilised or resolved. You have experienced a serious side effect You are unable to complete the visits, medication or trial documentation as required The trial doctor feels you no longer appear to benefit from the treatment
- What if there is a problem?
-
Any complaint about the way you have been dealt with during the trial or any possible harm you might suffer will be addressed. If you have any concerns about any aspect of this trial you should speak to your trial doctor who will do their best to answer your questions.
In the event that something does go wrong and you are harmed by taking part in the research and this is due to someone’s negligence then you may have grounds for legal action for compensation against Cambridge University Hospitals NHS Foundation Trust (or your hospital – for multicentre trials). If your claim is successful, your legal costs will be met. The normal National Health Service complaints mechanisms will still be available to you (if appropriate).
The NHS does not provide no-fault compensation i.e. for non-negligent harm, and NHS bodies are unable to agree in advance to pay compensation for non-negligent harm. They are able to consider an ex-gratia payment in the case of a claim. If you wish to complain or have any concerns about any aspect of the way you have been approached or treated during this trial, you can do this through the NHS complaints procedure. In the first instance, it may be helpful to contact the Patient Advice and Liaison Service (PALS)) at your hospital.
- Will my taking part in this trial be kept confidential?
-
For participants recruited at Cambridge University Hospital (where the Sponsor is also the site): Cambridge University Hospitals NHS Foundation Trust (CUH) is the Sponsor for this clinical trial based in the UK. They will be using information from you and your medical records in order to undertake this trial and will act as the data controller for this trial. This means that they are responsible for looking after your information and using it properly. The Sponsor organisation(s) will keep identifiable information about you for 5 years after the trial has finished to ensure your safety and allow the trial to be reviewed by the authorities after it is finished.
Your rights to access, change or move your information are limited, as the Sponsor organisation needs to manage your information in specific ways in order for the research to be reliable and accurate. To safeguard your rights, we will use the minimum personally-identifiable information possible.
You can find out more about how the Sponsor uses your information using the information below:
For Cambridge University Hospitals NHS Foundation Trust, please visit:
https://www.cuh.nhs.uk/corporate-information/about-us/our-responsibilities/looking-after-your-information or email the Data Protection Officer at gdpr.enquiries@addenbrookes.nhs.uk
Cambridge University Hospitals will collect your name and contact details to contact you about this trial and make sure that relevant information about the trial is recorded for your care, and to oversee the quality of the trial. Individuals from the Sponsor and regulatory organisations may look at your research records to check the accuracy of this trial. Cambridge University Hospitals will pass these details to the Sponsor along with the information collected from you. The only people in the Sponsor organisation who will have access to information that identifies you will be people who need to contact you in relation to this trial and to audit the data collection process.
Cambridge University Hospitals will keep identifiable information about you from this trial for 5 years after the trial has finished.
For participants recruited at other participating sites:
Your hospital will keep your name, (NHS number) and contact details to contact you about this trial, and make sure that relevant information about the trial is recorded for your care, and to oversee the quality of the trial. Certain individuals from the Sponsor(s) and regulatory organisations may look at your medical and research records to check the accuracy of this trial. The Sponsor(s) will only receive information without any identifying information.
All information collected about you as a result of your participation in the trial will be kept strictly confidential. Your personal and medical information will be kept in a secured file and be treated in the strictest confidence.
Once you have agreed to participate in this trial you will be allocated a Trial ID Number. This is a unique trial number which will be used on all your trial documentation along with your date of birth. Your date of birth is considered to be personal information. We collect this personal information on trial documentation to help ensure that the data we receive as part of your trial participation is correctly allocated to you. By cross-checking these two unique references we can ensure the integrity of the data.
The people who analyse the information will not be able to identify you and will not be able to find out your name, or contact details. Only anonymous trial data, without any personal information, will be published at the end of the trial.
When you agree to take part in this trial, the information about your health and care may be provided to researchers running other research studies in this organisation and in other organisations. These organisations may be universities, NHS organisations or companies involved in health and care research in this country or abroad. Your information will only be used by organisations and researchers to conduct research in accordance with the UK Policy Framework for Health and Social Care Research.
This information will not identify you and will not be combined with other information in a way that could identify you. The information will only be used for the purpose of health and care research and cannot be used to contact you or to affect your care. It will not be used to make decisions about future services available to you, such as insurance. If you have taken part in a parallel biomarker COVID-19 study at your hospital, we may wish to exchange information from the TACTIC-E trial with these study doctors to further enhance our knowledge of how the immune system handles COVID-19 and responds to different treatments. All your details will be anonymised.
We will need to inform your GP of your participation in this trial so that any medical decisions made by your GP account for any treatment you are receiving as part of this trial. Your GP may also contact us if they have any concerns about your participation in this trial.
- What will happen to my samples?
-
Blood and serum samples that are collected in this trial will be securely stored for the duration of the trial and will be accessible to authorised trial staff only. All trial samples will be labelled with your Trial ID and date of birth. During the trial, we will analyse your samples in a local laboratory though some may be analysed centrally in a Cambridge University Hospitals NHS Foundation Trust laboratory. With your permission, any unused samples at the end of this trial will be stored at Cambridge University Hospitals NHS Foundation Trust for future tests related to this study, and for future approved research projects.
- What about genetic tests
-
As part of the study, we may extract DNA from your sample. DNA is the chemical that makes up genes, influencing the factors we inherit and which determine our characteristics. We will also isolate and test other components of your blood such as RNA and protein and measure chemicals in the blood. We hope the results of this profiling will help us understand COVID-19 better. As this research is exploratory, you will not receive feedback regarding any ‘markers’ identified in your DNA.
- What will happen to the results of the trial?
-
The results of the trial will be anonymous and you will not be able to be identified from any of the data produced. When the results of this trial are available, they may be published in peer-reviewed medical journals and used for medical presentations and conferences. They will also be published on the EU Clinical Trials Register website, a central registry for all clinical trials conducted in the EU.
Coded datasets from the trial may also be made available to other researchers in line with national and international data transparency initiatives. These researchers may be outside the European Union and EEA zone where privacy laws may not be as stringent – however, none of your personal details will be sent outside the EU. If you would like to obtain a copy of the published results, please contact your trial doctor directly who will be able to arrange this for you.
- Who is funding the trial?
-
The trial is being funded by AstraZeneca and Evelo Biosciences Ltd.
- Who has reviewed this trial?
-
All research within the NHS is reviewed by an independent group of people called a Research Ethics Committee, to protect your interests. This trial has been reviewed and given favourable opinion by West Midlands - Coventry & Warwickshire Research Ethics Committee. The Medicines and Healthcare Products Regulatory Agency (MHRA) who are responsible for regulating medicines in the UK have also reviewed this trial.