Multi-Arm Therapeutic Study in Pre-ICU Patients Admitted with COVID-19
The COVID-19 pandemic, as declared on 11th March 2020 by World Health Organisation (WHO), is caused by a novel coronavirus (SARS-CoV-2). To date, it has caused over 40,000 deaths in the UK and over 400,000 documented deaths worldwide. The subset of people who develop severe COVID-19-related disease has a heightened immune response leading to organ damage.
The TACTIC programme emerged from the hypothesis that immunomodulation would be likely to reduce the severity of COVID-19-related disease – a model supported by results from the RECOVERY trial indicating that a corticosteroid, dexamethasone, reduces mortality in patients with severe disease. TACTIC has been designed to assess selected medications which modify aspects of the immune response. These medications have been chosen by a consortium of clinicians and clinician-scientists with expertise in the treatment of immune-mediated disease and it is hoped that they will further reduce morbidity and mortality from COVID-19-related disease.
The TACTIC programme uses a platform design which provides flexibility to swap out and add in alternative treatments depending on whether the data show:
- Effectiveness - in which case the treatment should be moved into NHS clinical care
- Ineffectiveness or safety concern - in which case the treatment should be withdrawn from the study
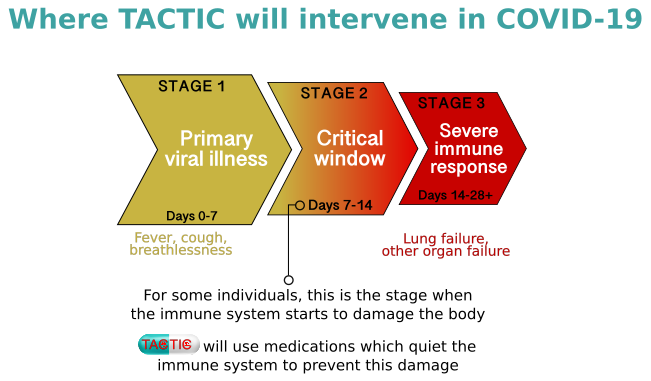
Where TACTIC will Intervene
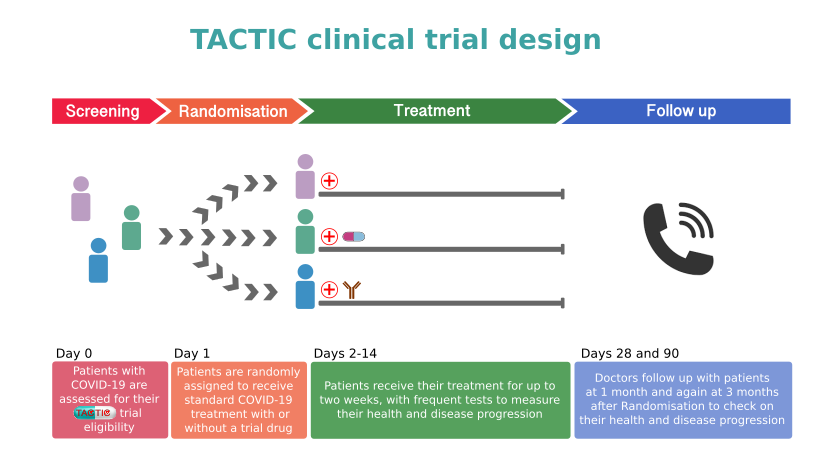
TACTIC Clinical Trial Design
There are two trials in the TACTIC programme: TACTIC-R and TACTIC-E. TACTIC-R is testing existing drugs (known as re-purposing) which are already used to block two distinct immune response pathways. Meanwhile, TACTIC-E looks at the use of two new (experimental) treatments which are capable of modifying immune response pathways in humans such as those seen in COVID-19-related disease. The medications will be compared to a patient group receiving standard supportive care.
The following videos are part of the ‘COVID and Me’ series of seven short dramas about people’s experiences of COVID-19 research. These fictional pieces of drama aim to:
- Help members of the public understand and appreciate the role that clinical trial research will play in ending COVID-19 pandemic
- Explain the process of giving your permission to take part
By encouraging many different communities to take part we can ensure we find a treatment for everyone - but in order to do this, everyone must take part.
Varsha – crossing the line
Ed – carrots and marigolds
Kirsty – an awkward fit
Asif – shoulder to shoulder
Ollie – important person
Ife – chicken pepper soup
Trudy – the call